More than 500 million people worldwide suffer from obesity. Obesity is a complex medical disease that is poorly understood. Scientists have not yet established a biological cause for obesity. Obesity has a strong genetic component involving several genes that regulate body weight, appetite and energy metabolism. However, the identification and characterization of a cause effect relationship between certain gene expression and obesity in humans has remained elusive until few days ago.
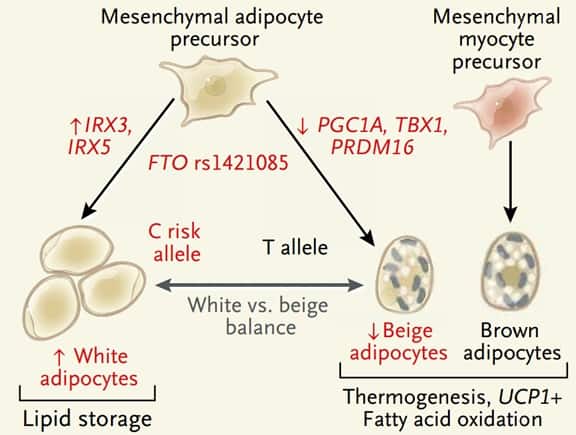
A new study published in the New England Journal of Medicine, demonstrates a causal relationship between a gene variant and fat accumulation in adipocytes. This fascinating article by Claussnitzer and colleagues is titled FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. FTO gene has been associated with obesity but so far FTO mechanism of action has not been understood. The authors propose that certain variants of the FTO gene change the basic function of human adipocytes from fat storage to fat burning. When exposed to a high-fat diet, adipocytes in white adipose tissue store fuel as triglycerides for later use. In contrast, brown adipocytes in the inter-scapular regions of infants, young adults, and some older persons generate heat by burning fat. A third type of adipose tissue, often called “beige,” or “brown-like” fat, is found in some white adipose tissue depots. Beige adipocytes like the brown ones have thermogenic properties. They can burn fat. It has been hypothesized that obese persons have fewer beige adipocytes and therefore are primed to gain weight on high-fat diets.
By showing that FTO gene controls adipocyte development into either a white (fat accumulating cell) or beige (fat burning cell), this original study explains the strong genetic association between obesity and FTO gene. The study offers a potential biologic pathway that leads to fat accumulation in adipocytes. Future research will focus on how FTO, which is highly expressed in several tissues, can affect other organs. The creation of a knock-in mouse with high-risk FTO alleles should facilitate the determination of the contribution of these gene variants to obesity. It would be interesting to study gastric bypass or gastric sleeve surgery in a knock-in mouse model with high-risk FTO alleles. Will the surgery be less effective? Or will the neuro-hormonal changes induced by weight loss surgery trump the FTO induced reduction in energy metabolism?
Our knowledge of obesity and weight loss surgery mechanism of action remains at its infancy. Obesity is a complex disease with many overlapping pathways that control appetite, weight and energy metabolism. It is quite amazing that a simple procedure like gastric sleeve can overcome all these pathways to achieve significant and durable weight loss. One cannot but wonder about the central role of the stomach and more specifically the gastric fundus in energy metabolism. The simple resection of gastric tissue, following gastric sleeve surgery, changes the interaction between ingested food and gastrointestinal tract. This change results in decreased appetite, weight loss and improved blood sugar control. Bile acid metabolism, colonic intestinal flora, gastro-intestinal motility as well as a number of hormones like GLP-1, PYY and Ghrelin levels are altered. How and why do these changes occur when the stomach is resected is still a mystery. Despite the obesity epidemic affecting millions of people, the only effective treatment available in the twenty first century is a surgery that we don’t understand how it works. This is no surprise as we are just starting to understand the role of the gastrointestinal tract as an endocrine organ. Bariatric surgery has been assumed to work by restriction and malabsorption for the past 50 years. Nutrient sensing and the resultant complex array of signals that reaches the brain, pancreas, liver and the rest of the intestines are very new concepts. Total deaths from diabetes alone are projected to rise by more than 50% in the next 10 years. Hopefully our understanding of gut physiology and its role in blood sugar regulation and energy metabolism will keep up with the rise of gut related disorders like diabetes and obesity. In future blogs I would like to share with you some of my views about the role of the stomach in nutrient sensing and processing and how Gastric fundus invagination, a novel weight loss procedure, is at the center of all this.