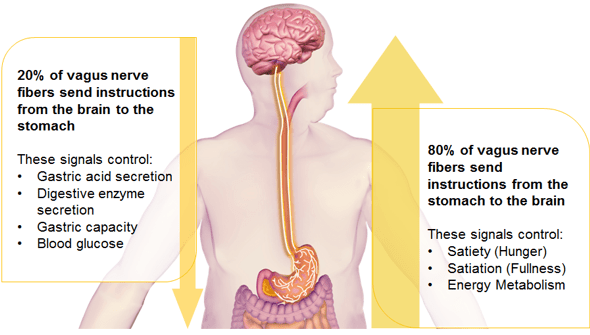
VBLOC therapy or intermittent vagal nerve blockade is a novel weight loss procedure that was recently approved by the FDA. The device is very unique as it targets the nervous connection between the brain and gastrointestinal system, the vagus nerve. Both anterior and posterior vagal trunks are encircled with special electrodes attached to a generator. The generator is implanted subcutaneously. Intermittent electrical pulses block the vagus nerve function. The vagus nerve is involved in gastric emptying and sending signals to the brain about stomach fullness. The specific mechanisms for weight loss due to the device are not known.
EMPOWER is a prospective randomized double blind controlled trial that enrolled around 500 patients with average BMI at 41. The main outcomes of the study were device safety and percent excess weight loss 12 months after implantation. The results showed no difference in weight loss between treated patients and controls and the device was found to be safe.
A similar clinical trial that included 233 patients with a BMI of 35 or greater found 8.5 percent more weight loss in treated patients compared to controls. The clinical study did not meet its original endpoint, which was that the experimental group loses at least 10 percent more excess weight than the control group. Still the FDA approved the device claiming that the benefits of VBLOC outweighed its risks. Additionally, the FDA sponsored a survey relating to patient preferences of obesity devices. The survey showed that some patients accept risks associated with VBLOC for the amount of weight loss expected to be provided.
In other words, the FDA approved a device for weight loss of questionable efficacy and unknown mechanism of action based on a patient survey. Patients are encouraged to actively participate in their medical care especially when it relates to obesity and bariatric surgery. However, patients need to be clearly and fairly educated about the efficacy of different weight loss procedures. Newer weight loss procedures using endoscopic techniques like intra-gastric balloon or neuro-modulation like VBLOC don’t seem to be effective weight loss solutions. Obese patients with type-2 diabetes and other obesity related comorbidities are unlikely to benefit from such interventions. Patients interested in significant and durable weight loss are most likely not going to be successful having a balloon in their stomach for 6 months. I am concerned for bariatric and acid reflux patients falling victims of inaccurate advertisement and exaggerated expectations. I hope the FDA would exert more caution before approving these devices.